Lithium-ion batteries: unveiling the role of oxygen
The democratization of electric vehicles has significantly increased the demand for lithium-ion batteries. Optimizing their autonomy, lifespan, and charging speed is therefore essential. These performances depend notably on the electrodes, where redox reactions take place, enabling the storage and release of energy in batteries. Among recent advances, the role of oxygen in these electrodes has become an essential issue and a source of controversy among researchers. A recent study led by Xu Gao from the Solid-State Chemistry and Energy Laboratory at Collège de France (CSE, CNRS/Collège de France/Sorbonne Université) sheds new lights on these debates by providing new insights into the chemical phenomena related to oxygen in these materials.
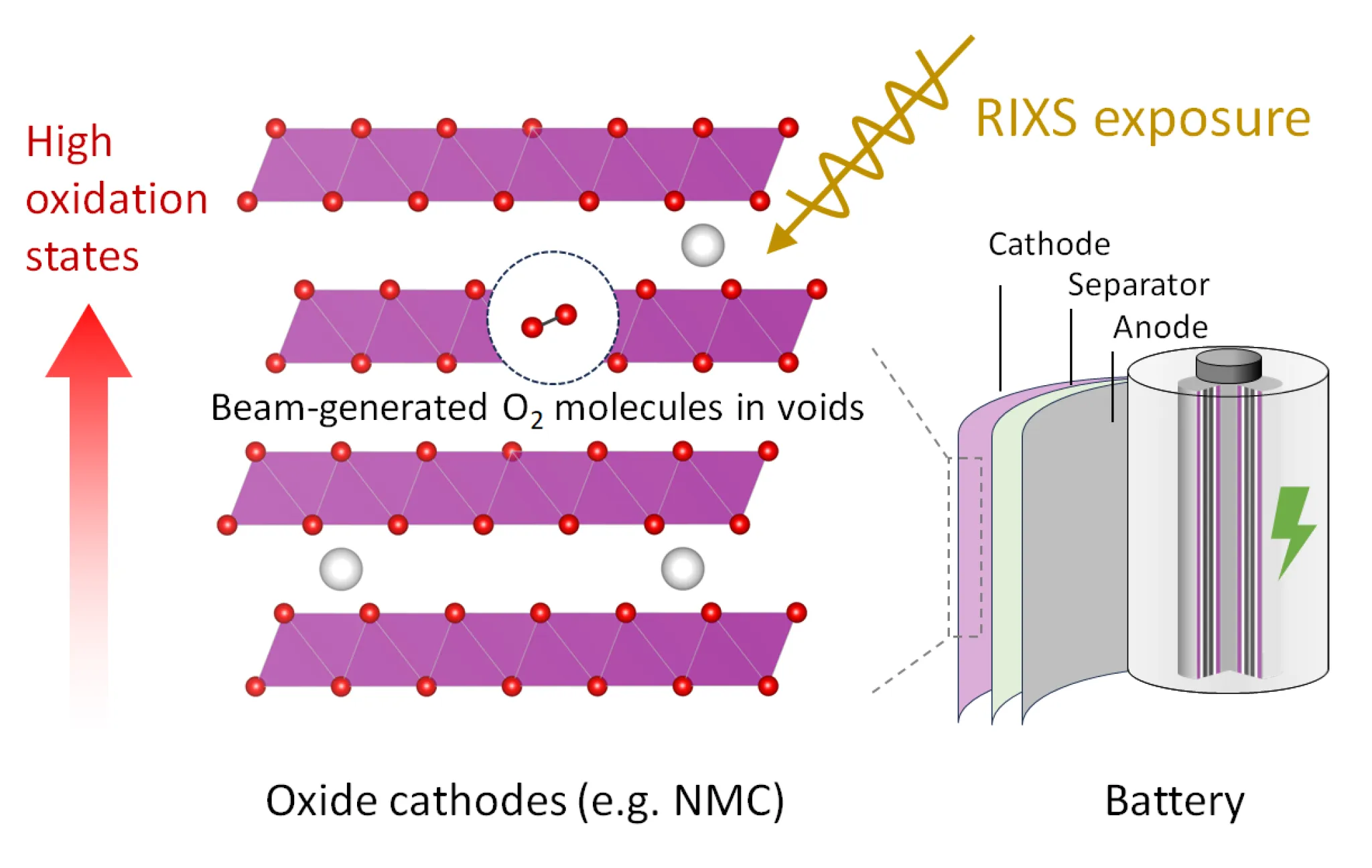
Recently, researchers have detected, through spectroscopic studies, the formation of trapped molecular oxygen (O₂) in lithium-rich electrodes. Their analyses led them to hypothesize that this species could be responsible for undesirable phenomena such as voltage fade, low energy efficiency, and even material degradation. However, these findings, published in prestigious journals (Nature, Science, Joule, …), have been the subject of controversy. This lack of consensus raised questions regarding the interpretation of the electrochemical mechanisms at play in electrodes and the actual impact of oxygen.
To resolve these uncertainties, Xu Gao and his collaborators conducted an in-depth analysis using resonant inelastic X-ray scattering (RIXS) at the European Synchrotron Radiation Facility (ESRF). Their results, confirmed by theoretical approaches, reveal that the molecular-oxygen-related signal is not exclusive to lithium-rich oxide electrodes. In fact, it is also detected in stoichiometric materials – those in which oxide and lithium are present in equal proportions –currently used in electric vehicles. These findings challenge the idea that trapped molecular oxygen is generated by the electrochemical reactions in batteries and that it is responsible for voltage fade or limited energy efficiency. Instead, the researchers conclude that O₂ formation is assisted by the interaction between the material and the RIXS-ray beam used in the experiment. In other words, the detection method itself generates molecular oxygen, explaining the discrepancies observed across different studies.
By clarifying the origin of molecular oxygen, this study helps to resolve a persistent confusion and redirect research toward more relevant approaches for improving batteries. Rather than focusing on trapped oxygen, efforts could be redirected toward studying interactions between oxygen and electrodes to optimize their stability.
These findings highlight the importance of methodological rigor and cautious interpretation of experimental results. They offer a new understanding of the mechanisms at work in lithium-ion batteries and open new perspectives for the development of more efficient and durable energy storage systems for electric mobility.
By Chloe Simha
Source : Xu Gao et al., Clarifying the Origin of Molecular O2 in Cathode Oxides, Nature Materials, (2025).
DOI: 10.1038/s41563-025-02144-7.

Xu Gao : Understanding Electrochemistry to Shape the Batteries of Tomorrow
Xu Gao became interested energy storage during his studies in China, through a project on lithium iron phosphate (LFP) batteries. Fascinated by this booming field, he later joined the Collège de France to collaborate with Professor Jean-Marie Tarascon. “I read many of his papers during my PhD, and wanted to learn how to conduct top-level research,” he explains.
Adapting to research in France brought some challenge: “It took me about six months to feel like I was meeting the high standards expected here.” However, this change in environment proved enriching. He particularly noticed the differences in how scientific problems are formulated and approached.
Xu Gao’s current work focuses on better understanding the fundamental chemical mechanisms at play in lithium-ion batteries. He believes that future efforts for this type of batteries should prioritize sustainability considerations rather than performance, which is already well optimized. He also emphasizes the importance of scientific collaboration in addressing economic and industrial challenges. “In research, we push the limits of science through collaboration, but in industry, competition can either promote or hinder progress. We need to find better ways to work together.”
His latest paper challenges established assumptions about the role of oxygen in batteries. “Our study goes against the general opinion and does not take previous claims for granted.” He hopes this work will spark new discussions and contribute to a deeper understanding of electrochemical mechanisms in lithium-ion batteries.